BRUKINSA® (zanubrutinib) is indicated for the treatment of adult patients with Waldenström’s macroglobulinemia (WM).1
Head-To-Head Study Of Brukinsa vs IBRUTINIB1
The safety and efficacy of BRUKINSA were evaluated in the ASPEN study, a Phase 3, randomized, open-label, multicentre study comparing BRUKINSA and ibrutinib in patients with treatment-naïve (TN) or relapsed/refractory (R/R) MYD88MUT WM.
ASPEN Study Design
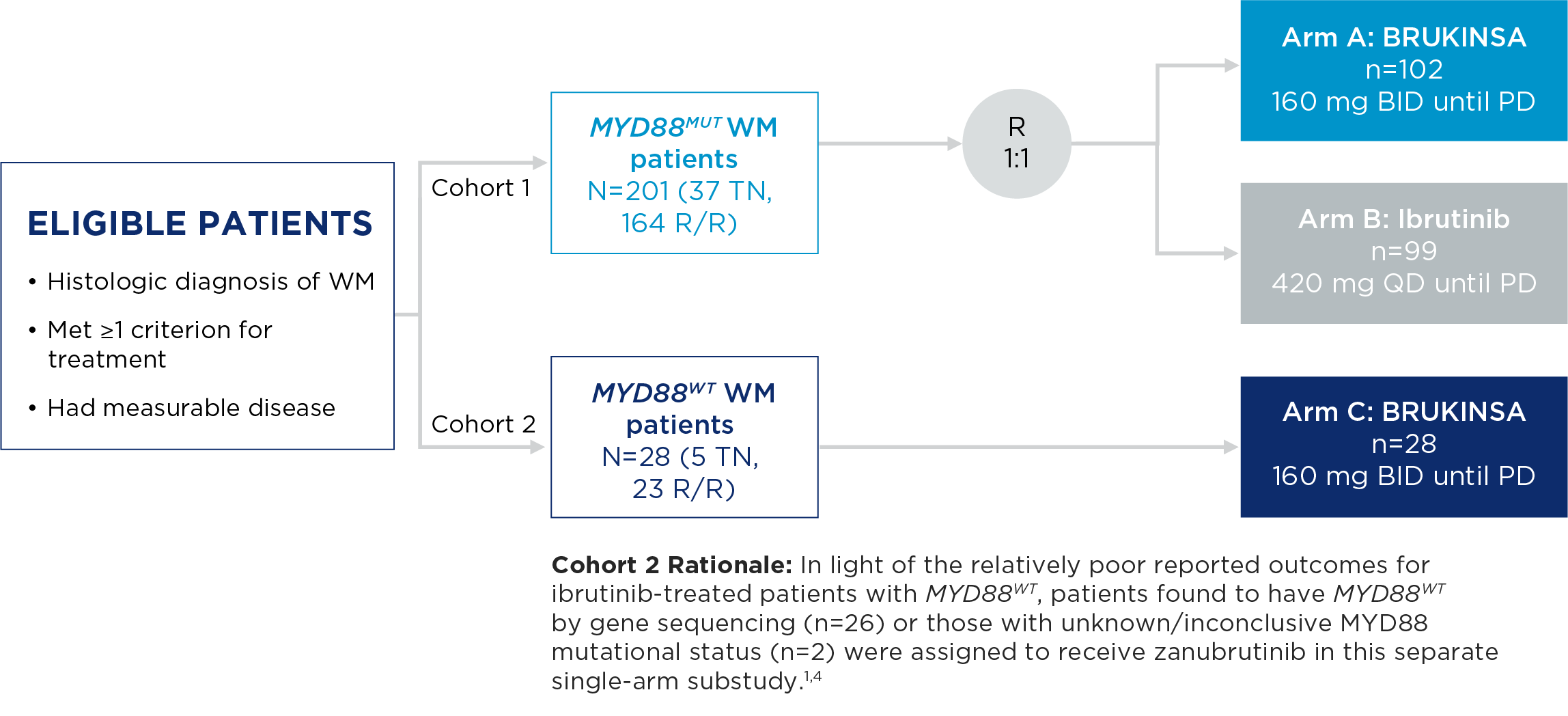
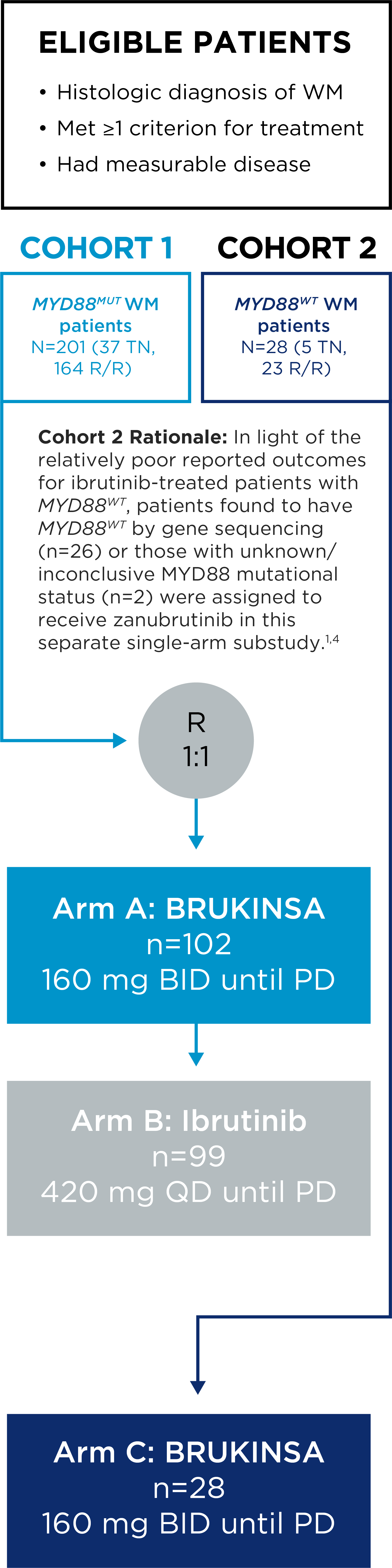
- The primary endpoint was rate of complete response (CR) or very good partial response (VGPR) in R/R MYD88MUT WM patients, as assessed by IRC.
Evaluated Globally in a Range of Patients
| Baseline Patient Characteristics1 | Cohort 1 MYD88MUT (n=201) |
Cohort 2 MYD88WT (n=28) |
|---|---|---|
| Median age | 70 years (range: 38-90) |
72 years (range: 39-87) |
| >75 years | 28% | 43% |
| Male | 67% | 50% |
| Caucasian | 91% | 96% |
| ECOG 0-1 2 |
94% 6% |
86% 14% |
| Treatment-naïve (TN) | 18% | 18% |
| Relapsed/refractory (R/R) | 82% | 82% |
| Median time from initial diagnosis | 4.6 years | 3.7 years |
| Median prior therapies | 1 (range: 1-8) | 1 (range: 1-5) |
- Patient disposition and demographics of patients in Cohort 1 were generally similar between BRUKINSA and ibrutinib arms except pertaining to percentage of patients >75 years of age (22% in the ibrutinib arm, 33% in the BRUKINSA arm).
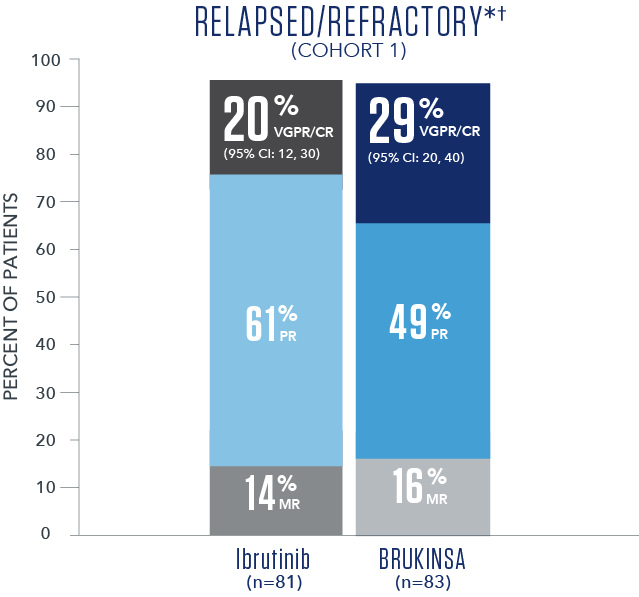
Efficacy results based on IRC assessment1
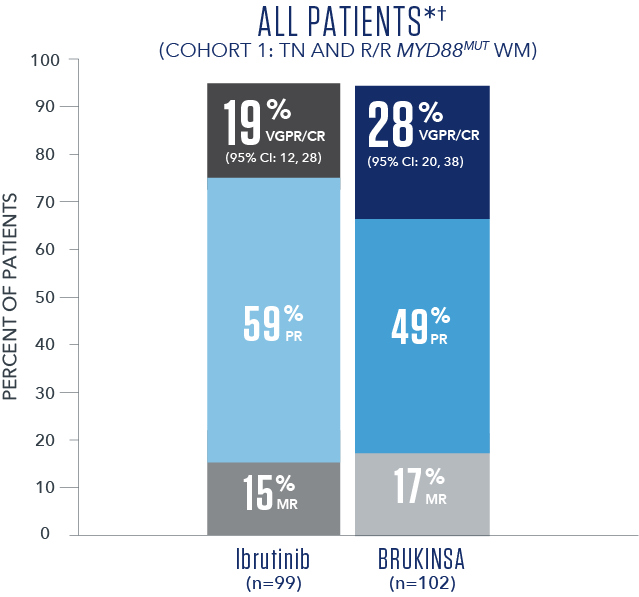
- * No CRs were observed.
- † Overall median follow-up time was 19.4 months.5
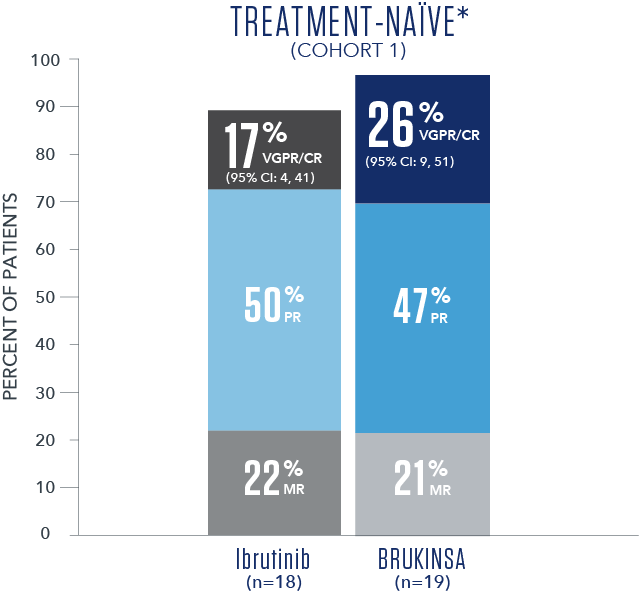
- * No CRs were observed.
- * No CRs were observed.
- † R/R median follow-up time was 18.8 months.
- The primary study results did not reach statistical significance in the R/R analysis set (2-sided p=0.12), thus the study did not meet the primary efficacy endpoint.
COHORT 2: VGPR/CR rates in MYD88 WT WM patients1*
Treatment-naïve
(TN)
20
Relapsed/refractory
(R/R)
29
- * No CRs were observed.
- BID=twice a day; ECOG=Eastern Cooperative Oncology Group; IRC=independent review committee; MR=minor response; PD=progressive disease; PR=partial response; QD=once a day.
Adverse Reactions (ARs) in ≥10% of patients with WM in COHORT 11
| System Organ Class | Adverse Reaction | BRUKINSA (N=101) | Ibrutinib (N=98) | ||
|---|---|---|---|---|---|
| All Grades (%) |
Grade 3 or Higher (%) |
All Grades (%) |
Grade 3 or Higher (%) |
||
| Blood and lymphatic system disorders | Neutropenia | 25 | 16 | 12 | 8 |
| Anemia | 12 | 5 | 10 | 5 | |
| Thrombocytopenia | 10 | 6 | 10 | 3 | |
| Cardiac disorders | Atrial fibrillation | 2 | 0 | 14 | 3 |
| Gastrointestinal disorders | Diarrhea | 21 | 3 | 32 | 1 |
| Constipation | 16 | 0 | 7 | 0 | |
| Nausea | 15 | 0 | 13 | 1 | |
| Vomiting | 9 | 0 | 13 | 1 | |
| General disorders and administration site conditions | Fatigue | 19 | 1 | 15 | 1 |
| Pyrexia | 13 | 2 | 12 | 2 | |
| Peripheral edema | 9 | 0 | 19 | 0 | |
| Infections and infestations | Upper respiratory tract infection | 24 | 0 | 29 | 1 |
| Nasopharyngitis | 11 | 0 | 7 | 0 | |
| Urinary tract infection | 10 | 0 | 10 | 2 | |
| Pneumonia | 9 | 3 | 20 | 7 | |
| Musculoskeletal and connective tissue disorders | Musculoskeletal pain | 30 | 7 | 24 | 0 |
| Pain in extremity | 11 | 1 | 7 | 0 | |
| Muscle spasms | 10 | 0 | 24 | 1 | |
| Nervous system disorders | Headache | 15 | 1 | 11 | 1 |
| Dizziness | 13 | 0 | 9 | 0 | |
| Renal and urinary disorders | Hematuria | 7 | 0 | 10 | 2 |
| Respiratory, thoracic, and mediastinal disorders | Dyspnea | 14 | 0 | 6 | 0 |
| Cough | 13 | 0 | 17 | 0 | |
| Epistaxis | 13 | 0 | 19 | 0 | |
| Skin and subcutaneous tissue disorders | Rash | 18 | 0 | 21 | 0 |
| Bruising | 18 | 0 | 34 | 0 | |
| Vascular disorders | Haemorrhage | 21 | 5 | 24 | 4 |
| Hypertension | 11 | 6 | 16 | 11 | |
The safety profile for Cohort 2 (patients with MYD88WT) was generally consistent with Cohort 1.
COHORT 1: BRUKINSA dose reduction and treatment discontinuation rates (N=101)1
Discontinuation rate
due to ARs*
4
Dose reduction
due to ARs†
14
- *The events leading to discontinuation were cardiomegaly, neutropenia, plasma cell myeloma, and subdural haemorrhage (1% each).
- †The most common adverse events leading to dose reduction were neutropenia (3%) and diarrhea (2%).
DOSING
ONCE- OR TWICE-DAILY DOSING1
The only BTKi that can be dosed either ONCE or TWICE daily1
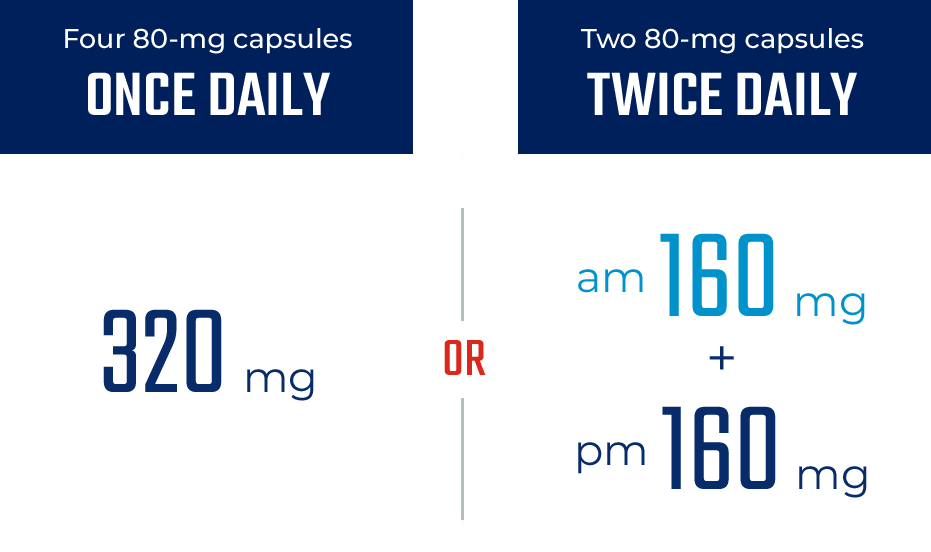
Administration1
- Can be taken with or without food. Can be taken with a high-fat meal – BRUKINSA drug concentration (AUC) is not affected
- Advise patients to swallow capsules whole with water—do not open, dissolve, or chew capsules
- BRUKINSA must not be taken with grapefruit juice, grapefruit, and/or Seville oranges
- If a dose of BRUKINSA is missed, it can be taken as soon as possible with a return to the normal schedule the following day
BRUKINSA should be taken until disease progression or unacceptable toxicity.
- AUC=area under the concentration-time curve.
RECOMMENDED DOSE ADJUSTMENTS1
| CYP | Coadministered drug | Recommended dose |
|---|---|---|
| Inhibition | Strong CYP3A inhibitor (e.g., posaconazole, voriconazole, ketoconazole, itraconazole, clarithromycin, lopinavir, ritonavir, telaprevir) | 80 mg once daily Interrupt dose as recommended for adverse reactions |
| Moderate CYP3A inhibitor (e.g., erythromycin, ciprofloxacin, diltiazem, dronedarone, fluconazole, verapamil, aprepitant) | 80 mg twice daily Modify dose as recommended for adverse reactions |
|
| Induction | Strong CYP3A inducer (e.g., carbamazepine, phenytoin, rifampin) | Avoid concomitant use; consider alternative agents with less CYP3A induction |
| Moderate CYP3A inducer (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin) | Avoid concomitant use. If concomitant use cannot be avoided, increase BRUKINSA dose to 320 mg twice daily. Monitor closely for toxicity. |
No dose adjustment is recommended in patients with mild to moderate renal or hepatic impairment.
Straightforward Dose Modification for ≥Grade 3 Adverse Reactions (ARs)1
Recommended dose modification for ≥Grade 3 ARs
- Grade ≥3: non-hematological toxicities
- Grade 3: febrile neutropenia/thrombocytopenia with significant bleeding
- Grade 4: neutropenia*/thrombocytopenia*
| Starting Dose | 1st Occurrence | 2nd Occurrence | 3rd Occurrence | 4th Occurrence |
|---|---|---|---|---|
| Start at 320 mg† total dose |
Interrupt BRUKINSA | Interrupt BRUKINSA | Interrupt BRUKINSA | Discontinue |
| Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
||
| No dose reduction. 320 mg† total dose |
Reduce to 160 mg total dose |
Reduce to 80 mg total dose |
- * Lasting >10 consecutive days.
- † 160 mg BID or 320 mg OD.
- ARs=adverse reactions; BID=twice a day; OD=once daily.
Pooled treatment-emergent adverse reactions in ≥10% of patients with hematologic malignancies* (N=1,550)6
| Treatment-Emergent Adverse Reactions | BRUKINSA (N=1,550) | |
|---|---|---|
| All Grades (%) | Grade ≥3 (%) | |
| Upper respiratory tract infection† | 37.1 | 2.2 |
| Bruising† | 30.4 | 0.5 |
| Neutropenia† | 27.5 | 18.5 |
| Haemorrhage/hematoma† | 27.4 | 3.0 |
| Musculoskeletal pain† | 27.0 | 1.8 |
| Rash† | 25.0 | 0.7 |
| Diarrhea | 18.8 | 1.5 |
| Cough† | 18.6 | 0.1 |
| Pneumonia† | 17.9 | 9.4 |
| Fatigue† | 16.2 | 1.2 |
| Thrombocytopenia† | 15.9 | 5.7 |
| Anemia† | 14.1 | 5.2 |
| Hypertension† | 13.0 | 6.5 |
| Constipation | 12.3 | 0.3 |
| Urinary tract infection† | 11.6 | 1.7 |
| Dizziness† | 10.7 | 0.3 |
- * Chronic lymphocytic leukemia, small lymphocytic lymphoma, Waldenström’s macroglobulinemia, mantle cell lymphoma, follicular lymphoma, marginal zone lymphoma, hairy cell leukemia, diffuse large B-cell lymphoma, and Richter’s transformation.
- † Indicates grouped term that includes multiple preferred terms.
Pooled selected treatment-emergent adverse events of special interest in patients with hematologic malignancies* (N=1,550)6
| Treatment-emergent adverse events | All Grades (%) | Grade ≥3 (%) |
|---|---|---|
| Arthralgia | 12.8 | 0.3 |
| Myalgia | 3.9 | 0.3 |
| Atrial fibrillation | 2.9 | 0.8 |
| Atrial flutter | 0.3 | 0.0 |
Of the 1,550 patients treated with BRUKINSA, 3.0% discontinued therapy and 5.2% experienced a dose reduction due to adverse reactions.1
BRUKINSA Ordering Information
BRUKINSA is available to all pharmacies in Canada. If you are a pharmacy looking to purchase BRUKINSA, it is available through the following wholesalers:
Sentrex order desk:
Email: orders@sentrex.com
Fax: 1-833-736-8739
Phone: 1-888-207-4618, Monday through Friday (8AM - 8PM ET).
Innomar order desk:
Email: specialtylogistics@innomar-strategies.com
Phone: 1-866-949-9927, Monday through Friday (8AM - 8PM ET).
 Patient Support
Patient Support
Designed to provide appropriate information and assistance to BRUKINSA patients, including:
Reimbursement/payment support
- Dedicated case managers to support insurance verification, bridge, and/or co-pay for patients to gain access to BRUKINSA
Education and support
- Dedicated nurse case managers for practices, patients, and caregivers
- Helps provide information about their disease and treatment with BRUKINSA
- Specialty pharmacists who speak to patients with every refill
Connections to third-party advocacy organizations
- Assists patients and caregivers with practical help through connections to advocacy groups and local/national free resources such as:
- Education and events
- Support group information
THE BEIGENE SAMPLE PROGRAM
BeiGene provides you with samples of BRUKINSA (zanubrutinib) for your patients

Receive a sample in 2 easy steps
- Click “Request Sample” below or request a sample form from your Regional Manager.
- Complete the form and submit.


These are prescription drug samples provided in accordance with Food and Drug Regulations.
It is unlawful to sell, trade, barter, return for credit, utilize to seek reimbursement, or bill patients for samples.


